Tirzepatide: The Most Effective Obesity Treatment Available
Eli Lilly's tirzepatide (Zepbound/Mounjaro) is the first dual GIP receptor agonist and GLP-1 receptor agonist—delivering up to 22% weight loss plus HbA1c reduction. Clinical trials show results that rival bariatric surgery. DEXA tracking ensures you're losing fat, not muscle. Starting at $199/month.


"I lost 87 lbs on GLP-1s—but I also lost muscle. That's why I track every patient's body composition with DEXA."
— Dr. Josh Lindsley, DO, DABOM · Board-Certified Obesity Medicine
What is Tirzepatide?
Tirzepatide from Eli Lilly is a breakthrough in endocrinology—the first FDA-approved dual GIP receptor agonist and GLP-1 receptor agonist. This dual mechanism improves insulin resistance, enhances weight management, and delivers superior results compared to single-agonist medications like semaglutide.
As a prescription medication from Eli Lilly and Company, it has FDA approval for obesity management and type 2 diabetes management to improve glucose control in eligible patients.
Its pharmacodynamics support hormonal regulation and blood glucose control, with strong glycemic control and a low risk of hypoglycemia for most patients.
The pharmacokinetics of tirzepatide allow once-weekly dosing via subcutaneous injection, delivered as a once-weekly injection. Clinical trials demonstrated not just weight loss but improved cardiovascular outcomes and metabolic markers. Patients on the highest dose lost an average of 22.5% of their body weight—52 pounds for someone starting at 230 lbs.
At Highland Longevity, we combine tirzepatide with DEXA body composition scans to ensure you're preserving lean muscle mass while losing fat—critical for long-term weight management success. For enhanced results, many patients add hormone optimization or peptide therapy to support metabolism and muscle preservation.

How Tirzepatide Works
Tirzepatide's dual mechanism targets two incretin pathways for enhanced weight loss.
Dual Receptor Action
Activates both GIP and GLP-1 receptors simultaneously, amplifying appetite suppression and metabolic effects beyond single-agonist medications.
Appetite Regulation
Signals the brain to reduce hunger and increase satiety, making it easier to eat less without constant willpower battles.
Metabolic Enhancement
Improves insulin sensitivity and blood sugar control while potentially increasing energy expenditure for greater fat burning.
Tirzepatide vs. Semaglutide
How the two leading GLP-1 medications compare in clinical trials. Learn more about semaglutide if you're weighing your options.
| Metric | Tirzepatide (Zepbound) | Semaglutide (Wegovy) |
|---|---|---|
| Average Weight Loss | Up to 22.5% | 15% |
| Patients Losing 5%+ | 96% | 86% |
| Patients Losing 10%+ | 84% | 69% |
| Patients Losing 20%+ | 57% | ~30% |
| Mechanism | Dual GIP + GLP-1 | GLP-1 only |
| Dosing | Twice weekly (split dose) | Once weekly |
Clinical Trial Results
The SURMOUNT clinical trials demonstrated unprecedented weight loss for a non-surgical treatment.
In patients with metabolic syndrome, trials report improved cardiometabolic markers, and cardiovascular benefits remain an active area of study.
Source: SURMOUNT-1 Clinical Trial (N Engl J Med 2022). Results with 15mg weekly dose over 72 weeks.
Real Patient Results
This is why we track body composition, not just weight. The scale doesn't tell the whole story.
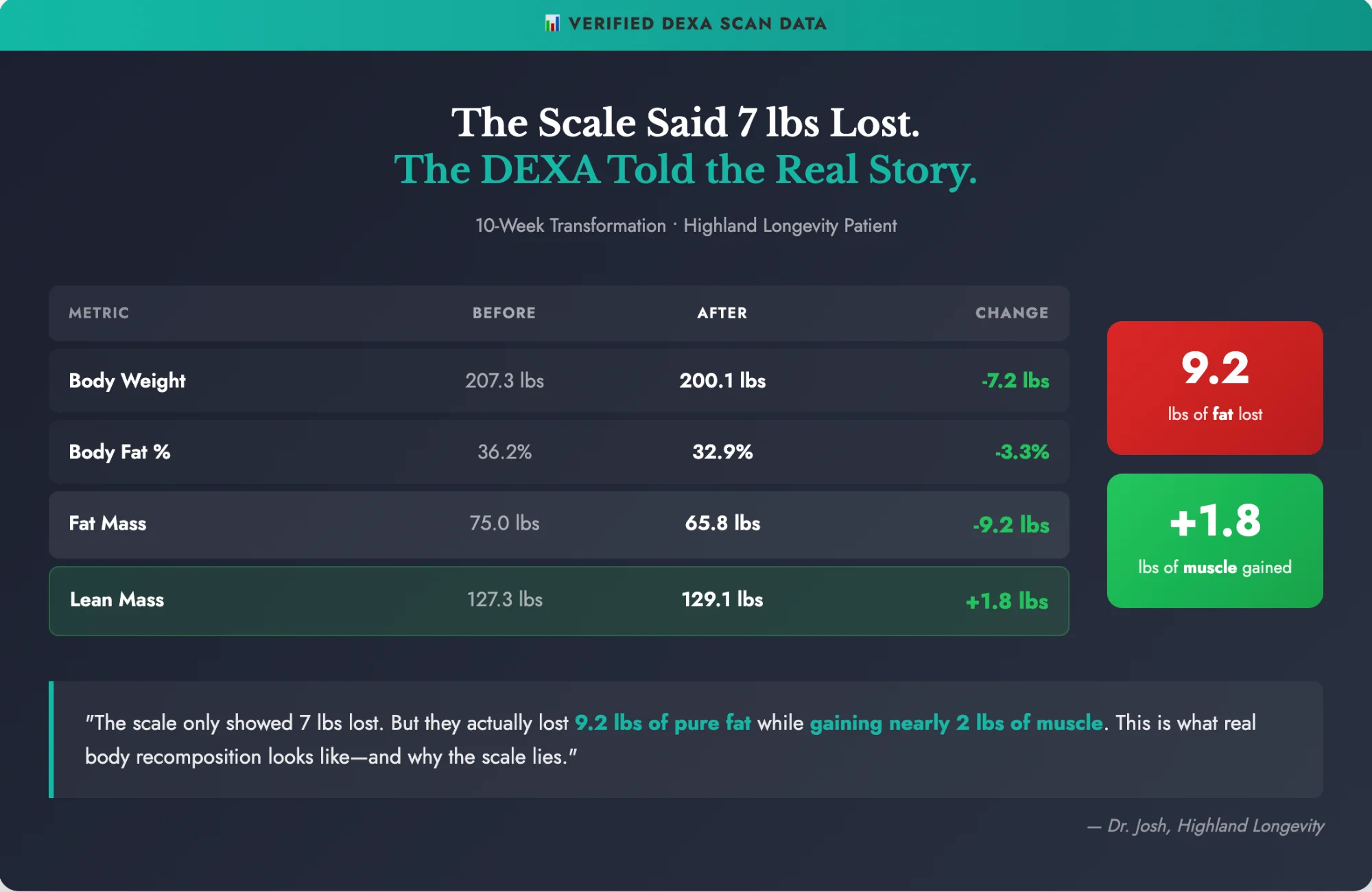
Verified DEXA scan data from Highland Longevity patient. Individual results may vary.
Dosing Schedule
We split doses into twice-weekly injections for better tolerability and steadier blood levels.
×2
Weeks 1-4
2.5mg/wk total
×2
Weeks 5-8
5mg/wk total
×2
Weeks 9-12
7.5mg/wk total
×2
Weeks 13-16
10mg/wk total
×2
Weeks 17-20
12.5mg/wk total
×2
Week 21+
15mg/wk total
Side Effects & Safety
Side effects are similar to other GLP-1 medications, and most adverse effects are gastrointestinal and improve over time.
Common Side Effects
- Nausea (improves after first few weeks)
- Diarrhea or constipation
- Decreased appetite (the intended effect)
- Dyspepsia (indigestion)
- Abdominal discomfort
When to Call Your Doctor
- Severe or persistent nausea/vomiting
- Signs of pancreatitis (severe abdominal pain)
- Gallbladder symptoms (right upper abdominal pain)
- Rapid heartbeat or dizziness
- Signs of allergic reaction
Is Tirzepatide Right for You?
Tirzepatide is FDA-approved for specific patient populations.
✓ Good Candidates
- BMI of 30 or higher (obesity)
- BMI of 27+ with weight-related conditions
- Looking for more aggressive weight loss than semaglutide
- Haven't reached goals with other GLP-1 medications
- Committed to lifestyle changes alongside medication
Not Recommended For
- Personal or family history of medullary thyroid cancer
- Multiple Endocrine Neoplasia syndrome type 2 (MEN2)
- History of pancreatitis or gallbladder disease
- Pregnant or planning to become pregnant
- Currently breastfeeding
Frequently Asked Questions
Clinical trials show up to 22.5% body weight loss at the highest dose (15mg) over 72 weeks. 96% of patients lose at least 5%, and over 50% lose 20% or more. Results vary based on individual factors, starting dose, and lifestyle adherence.
Both contain tirzepatide. Zepbound is FDA-approved specifically for weight loss. Mounjaro is approved for type 2 diabetes but is sometimes used off-label for weight loss. The medication is identical—only the indication and sometimes the dosing differ.
Tirzepatide is a dual agonist—it activates both GIP and GLP-1 receptors, while semaglutide only activates GLP-1. This dual action results in greater weight loss: up to 22.5% with tirzepatide vs. 15% with semaglutide in clinical trials.
It depends on your goals and medical history. Some patients start with semaglutide and switch to tirzepatide if they plateau. Others go straight to tirzepatide for maximum effect. We'll help you decide based on your situation during your consultation.
Studies show that stopping tirzepatide typically leads to weight regain—about two-thirds of lost weight returns within a year. This is why we emphasize body composition (preserving muscle), metabolic health, and sustainable nutrition habits alongside medication.
Coverage varies widely. Many insurers cover Mounjaro for diabetes but not Zepbound for weight loss. Some patients get coverage with prior authorization. We can help you navigate options including manufacturer savings programs.
Maximize Your Transformation
Many tirzepatide patients combine GLP-1 therapy with these services for comprehensive body recomposition.
Ready for Maximum Results?
Book a free consultation to discuss whether tirzepatide is right for you. We'll review your medical history, prior weight loss attempts, and goals to create your personalized plan.
Tirzepatide Near You
Serving the DFW metroplex from our Fort Worth clinic. Easily accessible from these surrounding communities.
Southlake
Serving Southlake, Grapevine & Colleyville
Keller
Serving Keller, North Fort Worth & Watauga
Westlake
Serving Westlake, Trophy Club & Roanoke
Colleyville
Serving Colleyville, Bedford & Euless
Trophy Club
Serving Trophy Club & surrounding areas
Roanoke
Serving Roanoke & surrounding areas
Haslet
Serving Haslet & the Alliance corridor
Medical References & Guidelines
- Jastreboff, A. M., et al. (2022). Tirzepatide Once Weekly for the Treatment of Obesity. The New England Journal of Medicine.
- Eli Lilly and Company. (2023). Zepbound (tirzepatide) injection Prescribing Information.
- Garvey, W. T., et al. (2023). Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet.